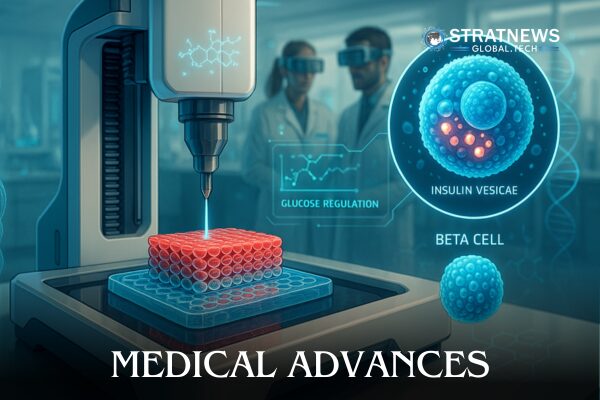
3D-Printed Human Pancreatic Cells Show Advances in Diabetes
Insulin-producing human pancreatic cells can now be manufactured using 3D printing, researchers announced at the International Transplant Congress in London. The team used a bio-ink made from human pancreatic tissue, stripped of cellular components, and alginate, a substance from seaweed, to print pancreatic islet cells.
These printed cells remained alive and functional for up to three weeks in laboratory tests, maintaining strong insulin responses to glucose. Unlike traditional methods that remove the structural support of cells, the bio-ink retains key extracellular matrix elements, enhancing cell survival and function.
The 3D-printed islets released insulin more efficiently in response to glucose and retained their structure without clumping or breaking down. Researchers believe this method could offer a safer and more comfortable alternative for patients with type 1 diabetes by enabling implantation under the skin using local anaesthesia.
Quentin Perrier of Wake Forest University said the findings mark a significant step towards creating an off-the-shelf diabetes treatment that may one day eliminate the need for insulin injections. Animal testing is now underway, with researchers exploring long-term storage options to expand accessibility.
Gene Editing Tool Targets Mitochondrial Disorders
New gene editing tools could pave the way for treating currently incurable mitochondrial disorders, laboratory experiments suggest. Mitochondria, the energy producers within cells, have their own DNA, and mutations can cause severe diseases affecting the brain, heart, muscles, and kidneys.
Traditional CRISPR tools have been ineffective due to challenges in entering mitochondria. However, researchers led by Dr Martijn Koppens at the University Medical Center Utrecht used a tool called DdCBE, which employs a bacterial toxin as molecular scissors, to edit mitochondrial DNA successfully.
Encapsulating the tool in lipid particles, similar to mRNA vaccine delivery, the team introduced and corrected mutations in liver cells and patient-derived cells with a genetic kidney disorder. Published in PLoS Biology, the findings could transform the development of therapies and accurate models for mitochondrial diseases.
Blood Thinners Safe Soon After Stroke, Study Finds
Starting blood thinners soon after a stroke caused by atrial fibrillation is safe and effective for most patients, according to a new analysis in The Lancet. Reviewing data from nearly 5,500 patients, researchers found that initiating direct oral anticoagulants like Xarelto and Eliquis within four days did not increase brain bleeding risks.
Previously, treatment was delayed due to concerns over bleeding. However, the study, led by Nick Freemantle from University College London, found that early initiation reduced the risk of recurrent strokes without increasing bleeding complications.
While early treatment is generally beneficial, an accompanying editorial noted the need for individual assessments, considering factors like age, medication use, and frailty when determining treatment timing.
These developments across diabetes care, genetic disorders, and stroke treatment highlight promising advances with the potential to improve patient outcomes and accessibility to effective therapies.
with inputs from Reuters